Case Study
Patient D,
Male, Nepalese
Table of Contents
Patient Overview
Age at time of treatment: 36 – 45
Injury Level: T7, T8
Treatment Received: Stem Cells, Epidural Stimulation
Location of Treatment: Thailand
Time between injury and treatment: 2 – 5 years
Date of Surgery: 12/07/2018
Date of Discharge: 16/08/2018
Condition on Admission
Patient sustained a traumatic spinal cord injury at T7-T8 level on May 31, 2015. His MRI scan showed T8 fracture dislocation with subsequent partial spinal cord myelomalacia (softening of the spinal cord). Patient is paraplegic, so fine motor skills in his hands and fingers are normal. Patient has loss of sensation and motor function in his bilateral lower limbs, and decreased mobility. Patient is able to stand with support, but for only a short period of time. He does not have a history of spasticity, severe spasms/spastic attacks, or neuropathic pain. Patient experiences bowel and bladder incontinence but is independent in his daily life activities.
Previous Therapies & Treatments
Patient received T6 – T10 pedicle screw fixation, plus fusion with a bone graft. He received analgesics, IV fluids, and physiotherapy treatment before being admitted to one of Verita Neuro’s partner hospitals. He experienced minor improvements in his condition following these interventions.
Patient sustained a traumatic spinal cord injury at T7-T8 level on May 31, 2015. His MRI scan showed T8 fracture dislocation with subsequent partial spinal cord myelomalacia (softening of the spinal cord). Patient is paraplegic, so fine motor skills in his hands and fingers are normal. Patient has loss of sensation and motor function in his bilateral lower limbs, and decreased mobility. Patient is able to stand with support, but for only a short period of time. He does not have a history of spasticity, severe spasms/spastic attacks, or neuropathic pain. Patient experiences bowel and bladder incontinence but is independent in his daily life activities.
Verita Neuro Treatment Received
After a spinal MRI scan, and comprehensive blood work, the patient underwent laminectomy and implantation of the epidural spinal cord stimulator on July 12th, 2018. The device is the ‘Medtronic Restore Advance 16-electrode MRI Compatible Device’. The surgery and post-operative care proceeded without significant adverse effects and no complications were reported during the hospital stay. There was normal healing of the surgical wound.
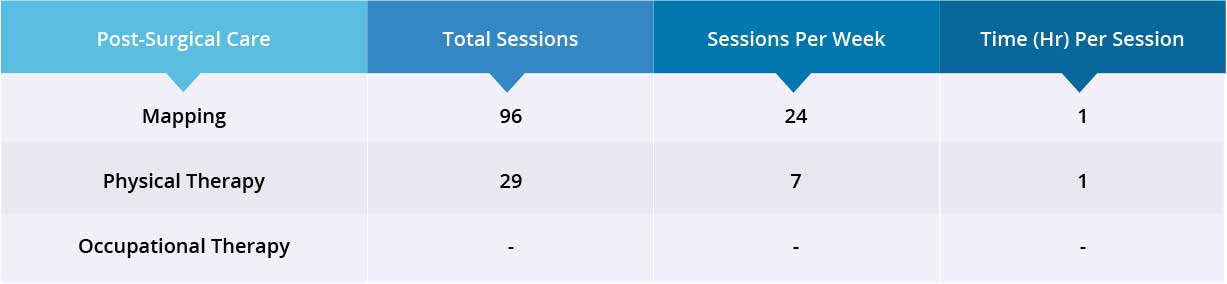
After epidural stimulation surgery, patient received 96 mapping sessions and 29 physical therapy sessions. Patient also received 40 million Mesenchymal Stem Cells (MSCs) through one IV injection and 80 million Amniotic Fluid Stem Cells (hAFSCs) through two lumbar puncture injections. All treatments, including lumbar puncture injections, went well without adverse effects and no short-term or acute complications were reported during the treatment.
Post-surgical device mapping and rehabilitation was carried out for 35 days, then the patient was discharged.
Results
- Motor Functions
- Sensory Functions
- Autonomic Functions
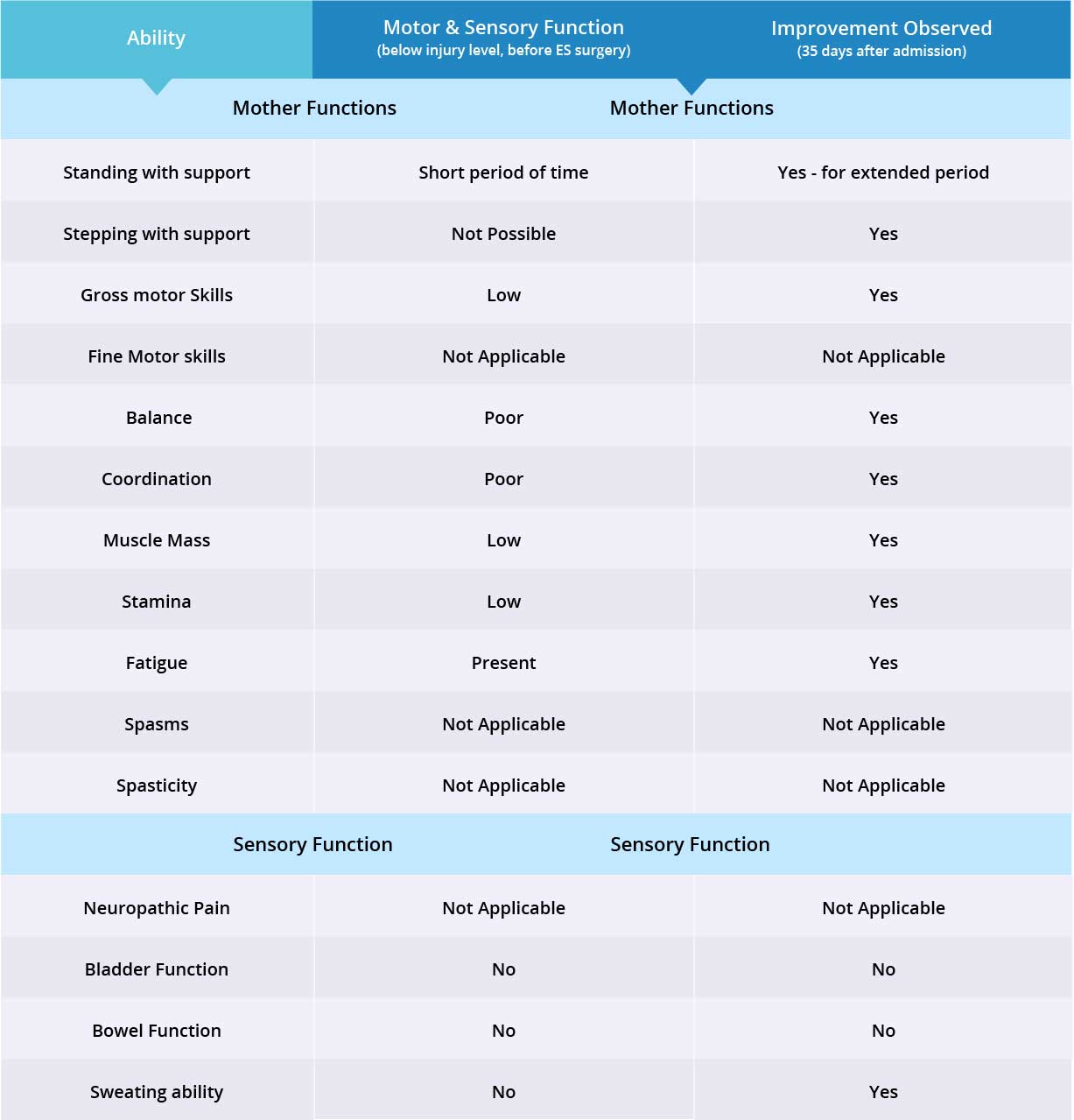
Improvements are monitored in 15 targeted areas: 11 Motor areas and 4 Sensory areas. However, the number of targeted areas may vary depending on patient’s condition prior to admission. If patient does not experience symptoms in certain Motor/Sensory functions, or is not impaired in a specific targeted area prior to surgery, it is excluded from the report (Not Applicable). If there is progress in any given area — either mild, moderate, or significant — it is measured and reported as positive (“Yes”). No improvement, the existence of pain or spasms, or an inability to perform a measured function is reported as “No”.
Results Interpretation
Patient is paraplegic with impairment in motor and sensory functions from only the waist down. Therefore fine motor skills were not measured for this case. Patient does not have a history of spasticity or neuropathic pain, so these have been excluded from the case report. Motor function improved in 8 of 8 targeted areas when the epidural stimulation device was switched on. Sensory function improved in 1 of 3 targeted areas. Overall improvement was seen in 9 of 11 of targeted motor and sensory function areas.
