This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Case Study
Patient F,
Male, Canadian
Table of Contents
Patient Overview
Age at time of treatment: 18 – 25
Injury Level: C5
Treatment Received: Epidural Stimulation
Location of Treatment: Thailand
Time between injury and treatment: 2 – 5 years
Date of Surgery: 25/06/2018
Date of Discharge: 21/07/2018
Condition on Admission
Patient sustained a C5 level spinal cord injury on November 12th, 2015, during a hockey game. Initial diagnosis of ASIA A score was changed to ASIA B within a few weeks of the trauma. Patient has no hand or leg function. Triceps and core strength were initially very weak, but have improved with time. Patient has neurogenic bowel and bladder function but is otherwise a healthy individual with no secondary injuries or diagnoses present.
Previous Therapies & Treatments
Patient had not received any surgery since his injury besides broken vertebrae repair. Patient received mesenchymal stem cells and allogenic stem cells, in the form of IV infusion and lumbar punctures, as well as physical rehabilitation. This resulted in minor improvements in his functions.
Patient sustained a C5 level spinal cord injury on November 12th, 2015, during a hockey game. Initial diagnosis of ASIA A score was changed to ASIA B within a few weeks of the trauma. Patient has no hand or leg function. Triceps and core strength were initially very weak, but have improved with time. Patient has neurogenic bowel and bladder function but is otherwise a healthy individual with no secondary injuries or diagnoses present.
Verita Neuro Treatment Received
After a spinal MRI scan, an EMG, and comprehensive blood work, patient underwent laminectomy and epidural spinal cord stimulator implantation on June 25th, 2018. The device is the ‘Medtronic Restore Advance 16-electrode MRI Compatible Device’. The surgery was completed without issue and no serious complications were reported during the postoperative hospital stay. Surgical wounds healed normally and no spinal cord or superficial wound infection was reported.
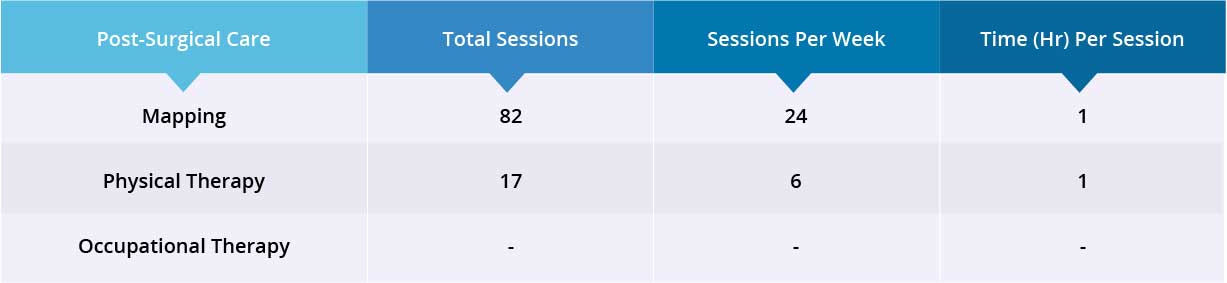
Results
- Motor Functions
- Sensory Functions
- Autonomic Functions
After 35 days, patient was discharged to continue his physiotherapy back home.
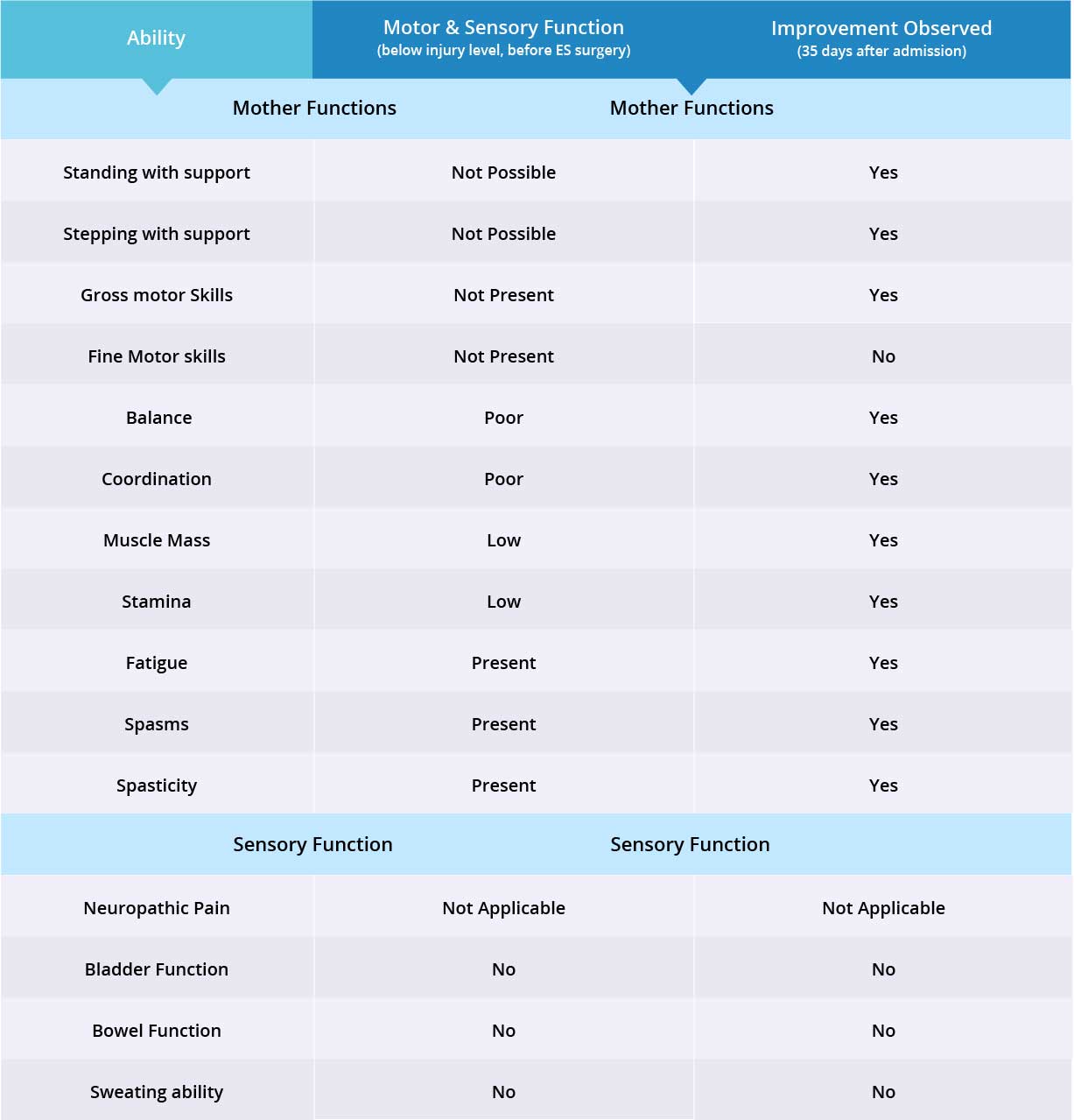
Improvements are monitored in 15 targeted areas: 11 Motor areas and 4 Sensory areas. However, the number of targeted areas may vary depending on patient’s condition prior to admission. If patient does not experience symptoms in certain Motor/Sensory functions, or is not impaired in a specific targeted area prior to surgery, it is excluded from the report (Not Applicable). If there is progress in any given area — either mild, moderate, or significant — it is measured and reported as positive (“Yes”). No improvement, the existence of pain or spasms, or an inability to perform a measured function is reported as “No”.
Results Interpretation
In this patient, neuropathic pain was not present, therefore 14 areas instead of 15 were reviewed. The primary treatment focus was to regain motor functions, which improved in 10 out of 11 targeted areas when the epidural stimulation device was switched on. Patient did not receive any stem cell injections therefore no improvements were visible in the 3 sensory function areas.
One Month Follow Up
- Motor Functions
- Sensory Functions
- Autonomic Functions
Balance and coordination improved moderately, and further improvements in muscle mass and stamina are anticipated with continued physical therapy. Patient can now sit up straight in his wheelchair and coordinate movement of his left and right foot during physical therapy. Patient is not able to lock knees yet and needs assistance in foot placement when stepping.
Six-Month Follow Up
Overall, patient is very satisfied with his outcomes after the Epidural Stimulation surgery. He will continue his physical therapy and another feedback call will be made in 3 months time.
Patient says “Since the treatment I’ve had new hope and energy towards my recovery. The epidural stimulator has been a great tool for exercise therapy and has had multiple benefits in my daily life. The potential to stand and take steps is extremely exciting. It keeps me motivated everyday and hopeful about the future.”
- Motor Functions
- Sensory Functions
- Autonomic Functions
He has noticed significant improvements during stepping training and is able to use a hoist to take assisted steps on a treadmill. Patient is able to lift his feet by himself and is more consistent and coordinated, but requires assistance in foot placement and locking his knees.
There is no visible change in the patient’s muscle mass, but he reports that his core muscles are getting stronger.
Patient also noticed that when the stimulator is on there is a decrease in spasms. However, during UTI, patient experiences bladder spasms and has less control when the Epidural Stimulation device is switched on. Patient has not seen any changes in neuropathic pain and is still taking the same dose of Nabilone.

