This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Case Study
Patient I,
Male, Lithuanian
Table of Contents
Patient Overview
Age at time of treatment: 26 – 35
Injury Level: C5
Treatment Received: Stem Cells, Epidural Stimulation
Location of Treatment: Thailand
Time between injury and treatment: < 1 year
Date of Surgery: 02/04/2018
Date of Discharge: 09/05/2018
Condition on Admission
Patient sustained a traumatic C5 spinal cord injury on June 4, 2017, characterized by incomplete quadriplegia and complete paraplegia. He has limited sensory and motor functions in the hands and arms and no voluntary motor function in his lower limbs. He suffers from neurogenic bladder and bowel, but does not suffer from significant spasms, spasticity, or neuropathic pain.
Previous Therapies & Treatments
Patient underwent tracheostomy and the tracheal tube was removed in September, 2017. Patient received spinal decompression surgery at C5 and the Aesculap “Spine System Evolution” titanium implant. Patient has been receiving therapy from a rehabilitation hospital in Lithuania.
Patient sustained a traumatic C5 spinal cord injury on June 4, 2017, characterized by incomplete quadriplegia and complete paraplegia. He has limited sensory and motor functions in the hands and arms and no voluntary motor function in his lower limbs. He suffers from neurogenic bladder and bowel, but does not suffer from significant spasms, spasticity, or neuropathic pain.
Verita Neuro Treatment Received
After a spinal MRI scan, EMG, and comprehensive blood work, patient underwent laminectomy and implantation of the epidural stimulation device at T12-L1 level. The device is the ‘Medtronic Restore Advance 16-electrode MRI Compatible Device’. He received intraspinal injection at the C5-C6 levels on April 2, 2018.
The surgery was completed without significant adverse events and no serious complications were reported during the postoperative hospital stay. Surgical wounds healed normally and no spinal cord or superficial wound infection was reported.
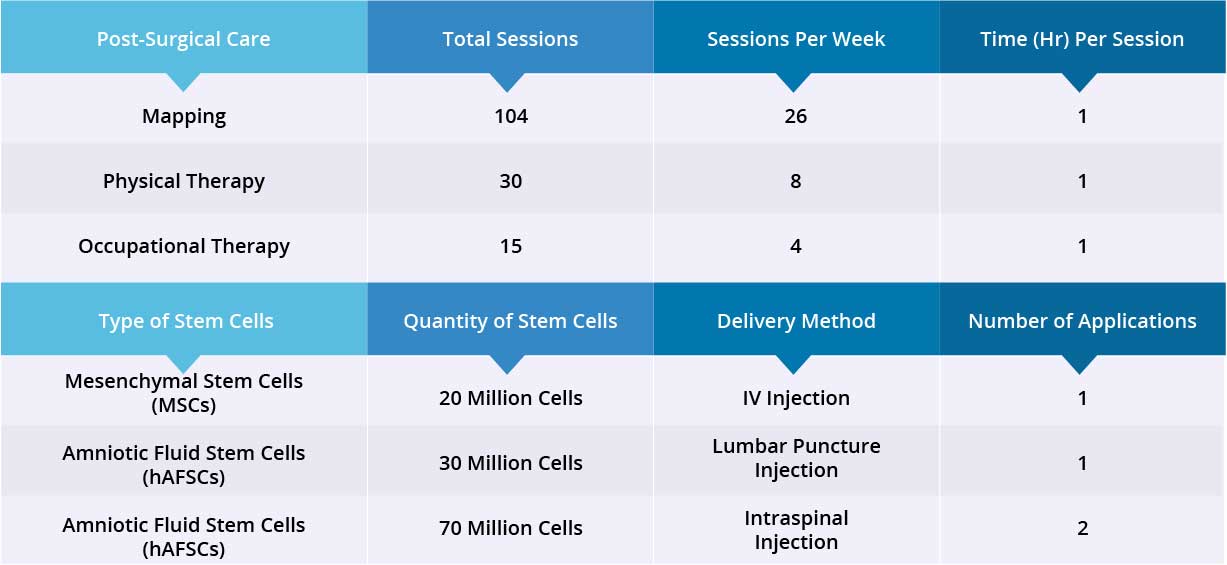
After epidural stimulation surgery, patient received 104 Mapping sessions, 30 Physical Therapy sessions and 15 Occupational Therapy sessions. Patient also received 20 million Mesenchymal Stem Cells (MSCs) via one IV injection, and 100 million Amniotic Fluid Stem Cells (hAFSCs) via one lumbar puncture injection and one intraspinal injection. All three applications went well without adverse effects and no short-term or acute complications have been reported.
Device mapping and therapy were carried out after surgery for 35 days, then patient was discharged.
Results
- Motor Functions
- Sensory Functions
- Autonomic Functions
Patient has fair static sitting balance with no back support needed and is able to sit up straight. Patient also demonstrates dynamic sitting balance, but loses balance when reaching out at an extended distance. Patient does not have static standing balance, and requires maximum assistance when changing position from sitting to standing, requiring a hoist for support.
Patient is able to stand with the support of a hoist, and requires assistance in locking knees and hips in both legs while standing. Patient has no trunk control while standing and is able to support only 50-70% of his weight on his legs when upright. Overall improvements in standing are very mild, but more feedback will be collected in 6 months time to see if any further improvements in standing have been made.
Due to the inability to stand effectively, stepping exercises were not conducted with this patient in his immediate post-surgery rehabilitation. However, a stepping program has been created so that patient is able to practice stepping during his physical therapy sessions back home.
Muscle mass and endurance were improved upon discharge.
1 Year Post-Treatment
One year after the initial treatment period with Verita Neuro, patient is performing 10 hours of physical therapy per week.
Gross motor functions have improved moderately. Patient is still able to flex and extend his right ankle, hips, and knees. The programs provided by Verita Neuro for gross motor function are still working very well. Patient feels stronger and is able to carry out more repetitions of kicking out his legs. From a scale of 1-to-5 with “1” being worse than before surgery and “5” being significantly improved from the surgery, the patient rates his gross motor functions somewhere between 3-4.
Patient is still practicing standing exercises and is able to stand without switching on the epidural stimulation device (with support). When the stimulator is switched on, the patient is able to stand bearing weight equally on either leg. He reports that he does not use the stimulator often. The patient is still not able to take steps or conduct gait training. Mild improvement was seen in patient’s static and dynamic sitting balance.
Patient reports that he has observed a decrease in his muscle mass, but has experienced a slight reduction in fatigue. We recommend this patient to come back for more mapping sessions to improve his stepping exercises. The patient should also increase the amount of physical therapy he conducts per week, and switch on the epidural stimulation device more often in order to gain muscle mass and improve more.
There was no noticeable improvement to his neurogenic bladder and bowel.
Patient received stem cell injections, therefore we expect to see results in these areas within 3 months time.
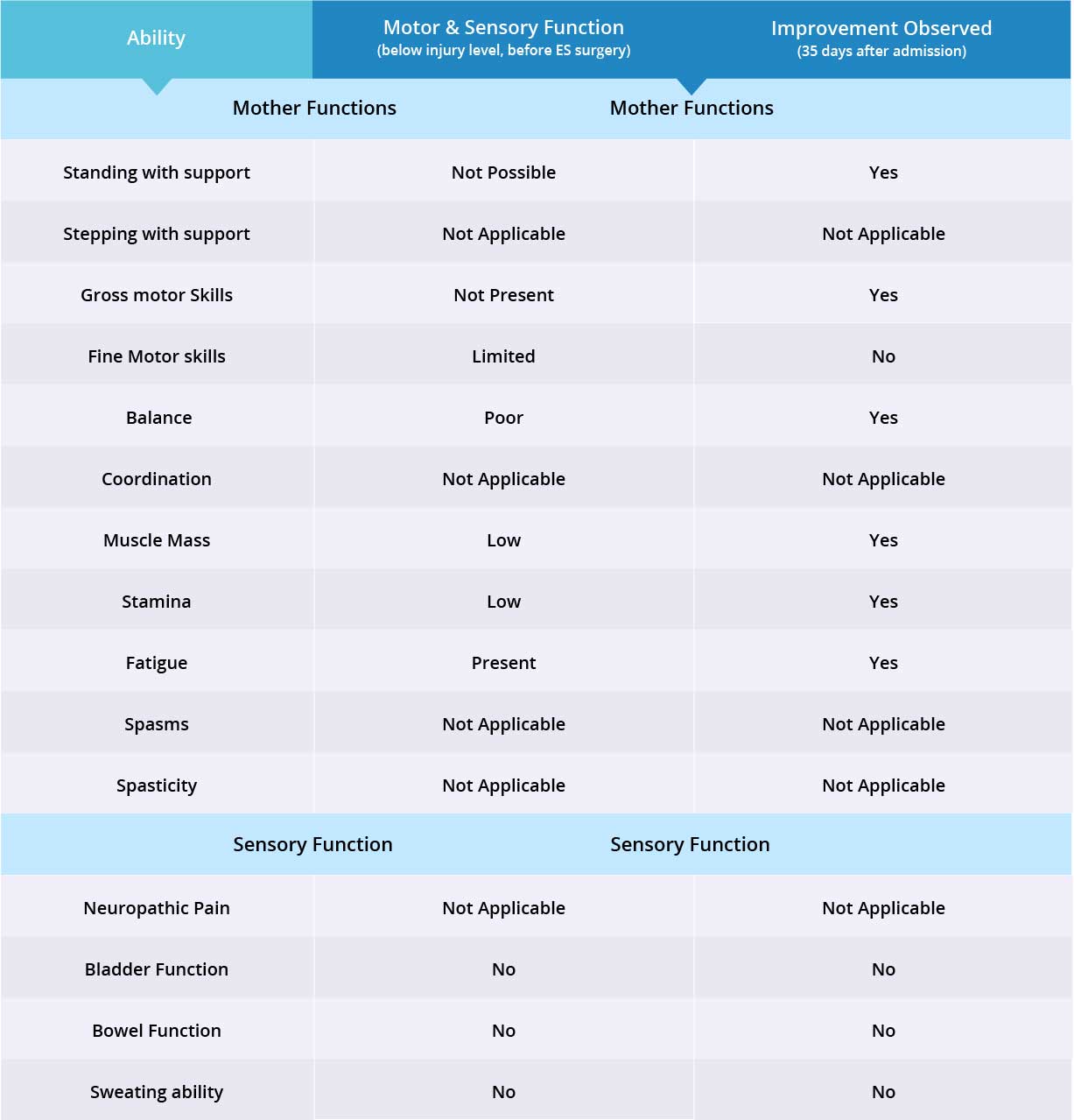
Improvements are monitored in 15 targeted areas: 11 Motor areas and 4 Sensory areas. However, the number of targeted areas may vary depending on patient’s condition prior to admission. If patient does not experience symptoms in certain Motor/Sensory functions, or is not impaired in a specific targeted area prior to surgery, it is excluded from the report (Not Applicable). If there is progress in any given area — either mild, moderate, or significant — it is measured and reported as positive (“Yes”). No improvement, the existence of pain or spasms, or an inability to perform a measured function is reported as “No”.
Results Interpretation
Stepping exercises were not conducted with this patient, therefore “stepping” and “coordination” are excluded from the report. Patient does not suffer from spasms or spasticity, so they are also excluded from the report. As a result, only 7 out of 11 motor function areas were measured, and there were improvements in 6 out of those 7 targeted areas when the epidural stimulation device was switched on.
Patient does not suffer from neuropathic pain, therefore only 3 out of 4 sensory function areas were measured. Patient has not experienced any improvements in the three measured sensory function areas, but more feedback will be collected after 6 months to note any improvements made by stem cell treatment. Overall, improvements were observed in 6 out of 10 targeted motor and sensory function areas.

