Case Study
Patient K,
Male, American
Table of Contents
Patient Overview
Age at time of treatment: Under 18
Injury Level: T6
Treatment Received: Stem Cells, Epidural Stimulation
Location of Treatment: Thailand
Time between injury and treatment: 5 – 15 years
Date of Surgery: 01/10/2018
Date of Discharge: 01/11/2018
Condition on Admission
Patient sustained a traumatic spinal cord injury at T6 level in November, 2012. Patient’s MRI scan showed T6-7 myelomalacia (softening of the spinal cord) and T6-9 syringomyelia (cyst in spinal cord). He has complete loss of motor and sensory functions below the injury level. He suffers from neurogenic bladder and bowel, and slight spasticity; he does not suffer from neuropathic pain. He is independent in his daily activities.
Previous Therapies & Treatments
Patient was admitted to UW Madison Children’s hospital for 6 weeks and then transferred to Froedtert Hospital (Milwaukee, USA) for another 4 weeks of rehabilitation.
Patient sustained a traumatic spinal cord injury at T6 level in November, 2012. Patient’s MRI scan showed T6-7 myelomalacia (softening of the spinal cord) and T6-9 syringomyelia (cyst in spinal cord). He has complete loss of motor and sensory functions below the injury level. He suffers from neurogenic bladder and bowel, and slight spasticity; he does not suffer from neuropathic pain. He is independent in his daily activities.
Verita Neuro Treatment Received
After a spinal MRI scan, EMG, and comprehensive blood work, patient underwent laminectomy, syringostomy, and implantation of the epidural stimulation device on October 1, 2018. The device is the ‘Medtronic Restore Advance 16-electrode MRI Compatible Device’. The surgery was completed without significant adverse events and no serious complications were reported during the postoperative hospital stay. Surgical wounds healed normally and no spinal cord or superficial wound infection was reported.
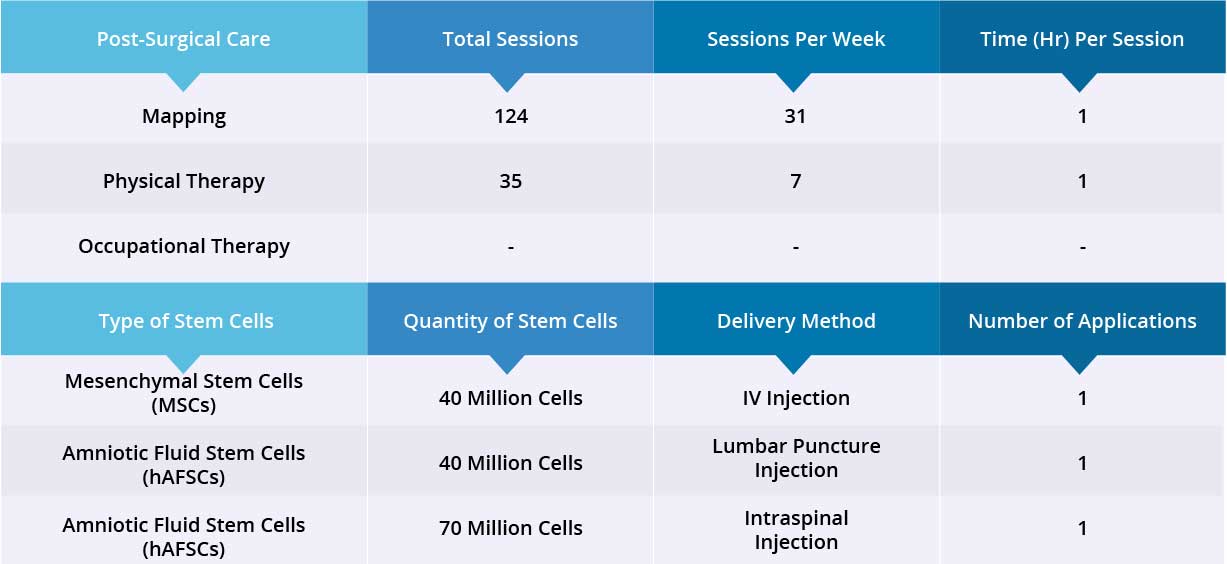
After epidural stimulation surgery, patient received 124 mapping sessions and 35 physical therapy sessions. Patient also received 150 million Stem Cells: 40 million Mesenchymal Stem Cells (MSCs) via one IV injection, and 110 million Amniotic Fluid Stem Cells (hAFSCs) via one lumbar puncture injection and one intraspinal injection. All three applications went well without adverse effects and no short-term or acute complications have been reported.
Device mapping and therapy were carried out after surgery for 35 days, then patient was discharged.
Results
- Motor Functions
- Sensory Functions
- Autonomic Functions
Patient has good static sitting balance, but does not exhibit dynamic sitting balance. When patient is standing during physical therapy sessions, he has poor static standing balance.
Patient is able to stand at the parallel bars with minimal assistance from physiotherapists. At certain times, requires assistance locking his knees while standing and is unable to keep them locked. He is able to lock his right knee better than his left knee. Patient has good trunk control while standing, but he bears weight more on the right side of his body than the left and requires assistance locking his hips.
Patient is able to take assisted steps with the support of a walking frame. During stepping exercises, patient is able to lift his leg up, but requires assistance in foot placement and with locking his hips and knees. Patient has good trunk control and fair coordination when alternating steps.
Muscle mass and endurance were improved upon discharge.
During mapping sessions, patient was put on a bowel program. It helped reduce the amount of time the patient spends emptying his bowel, from 45-60 minutes to 20-30 minutes daily, a significant improvement in quality of life.
There was no noticeable improvement to his neurogenic bladder and bowel.
Patient received stem cell injections, therefore we expect to see results in these areas within 6 months.
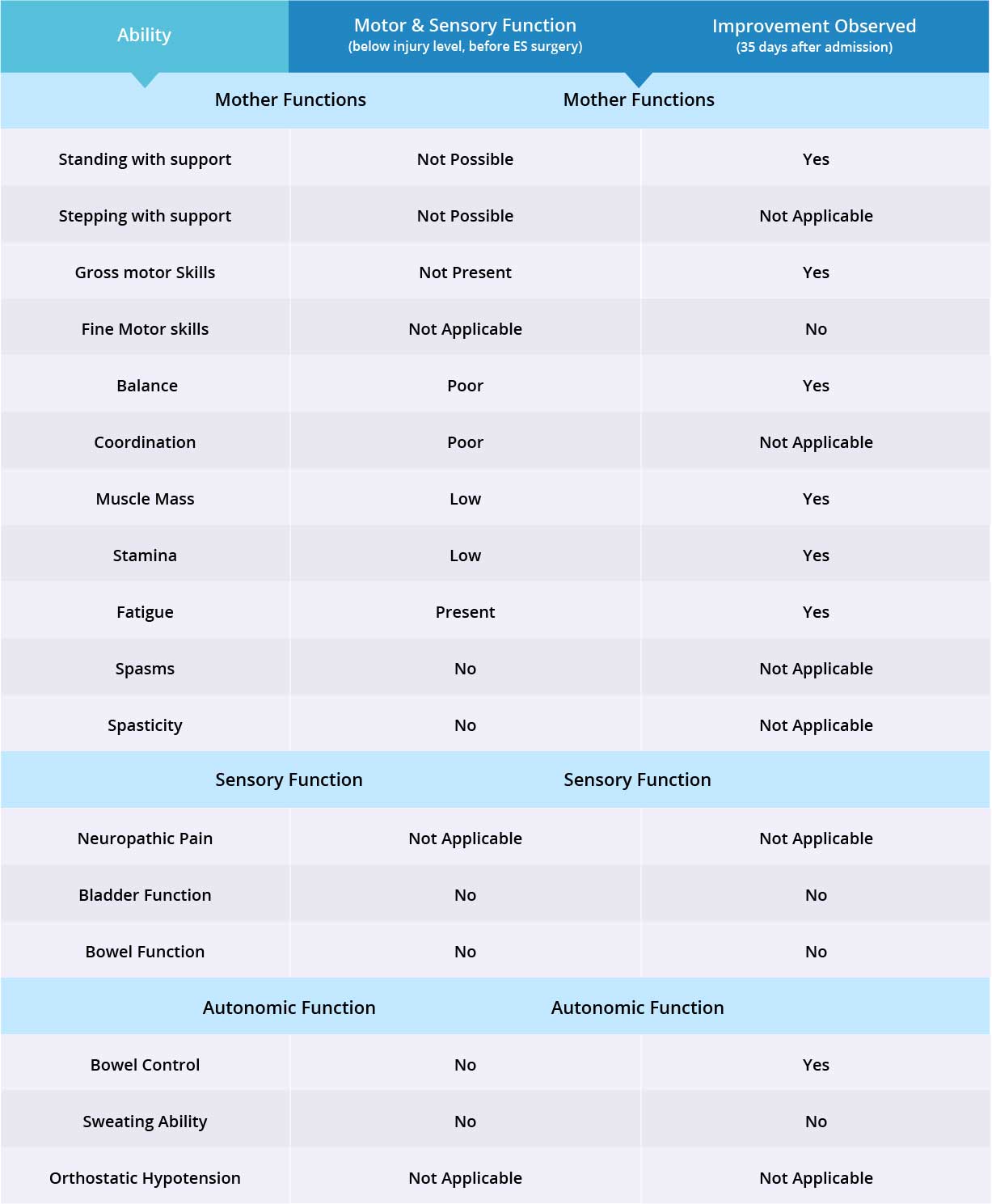
Improvements are monitored in 15 targeted areas: 11 Motor areas and 4 Sensory areas. However, the number of targeted areas may vary depending on patient’s condition prior to admission. If patient does not experience symptoms in certain Motor/Sensory functions, or is not impaired in a specific targeted area prior to surgery, it is excluded from the report (Not Applicable). If there is progress in any given area — either mild, moderate, or significant — it is measured and reported as positive (“Yes”). No improvement, the existence of pain or spasms, or an inability to perform a measured function is reported as “No”.
Results Interpretation
Fine motor skills were excluded from this case report since patient is paraplegic, therefore 10 out of 11 motor function areas were measured. Motor function improved in 8 out of 10 targeted areas when the epidural stimulation device was switched on.
Patient does not suffer from neuropathic pain, therefore 2 out of 3 sensory function areas were measured. Patient has not experienced any changes in those two sensory function areas, but more feedback will be collected after 3 months to note any improvements made by stem cell treatment.
Patient does not suffer from orthostatic hypotension, therefore 2 out of 3 autonomic function areas were measured. Patient’s bowel control improved when the epidural stimulation device was switched on. Overall, improvements were recorded in 9 out of 14 targeted motor, sensory, and autonomic function areas.
