Case Study
Patient M,
Male, British
Table of Contents
Patient Overview
Age at time of treatment: 26 – 35
Injury Level: T5, T6
Treatment Received: Stem Cells, Epidural Stimulation
Location of Treatment: Thailand
Time between injury and treatment: 2 – 5 years
Date of Surgery: 02/04/2018
Date of Discharge: 05/05/2018
Condition on Admission
Patient sustained a complete T5-6 fracture-dislocation spinal cord injury in 2014 with subsequent partial spinal cord transection and myelomalacia (spinal cord softening). Patient has minimal motor or sensory function below injury level and is suffering from neurogenic bladder and bowel. Patient does not experience severe spasms or spasticity and does not suffer from neuropathic pain. Patient is independent in his daily activities.
Previous Therapies & Treatments
N/A
Patient sustained a complete T5-6 fracture-dislocation spinal cord injury in 2014 with subsequent partial spinal cord transection and myelomalacia (spinal cord softening). Patient has minimal motor or sensory function below injury level and is suffering from neurogenic bladder and bowel. Patient does not experience severe spasms or spasticity and does not suffer from neuropathic pain. Patient is independent in his daily activities.
Verita Neuro Treatment Received
After a spinal MRI scan, EMG, and comprehensive blood work, patient underwent laminectomy and implantation of the epidural stimulation device on April 2, 2018. The device is the ‘Medtronic Restore Advance 16-electrode MRI Compatible Device’. The surgery was completed without significant adverse events and no serious complications were reported during the postoperative hospital stay.
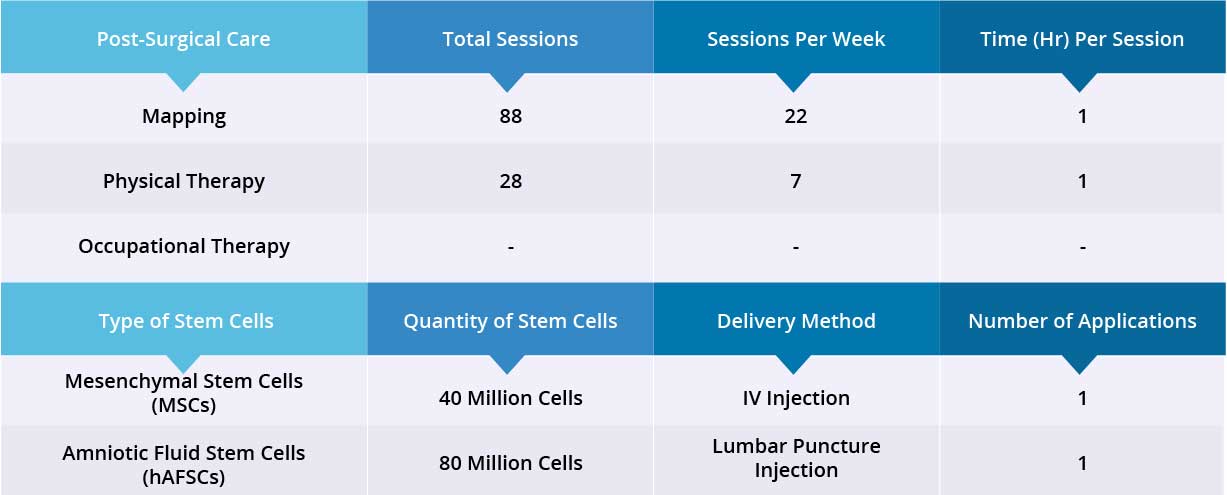
After epidural stimulation surgery, patient received 88 mapping sessions and 28 physical therapy sessions. Patient also received 120 million stem cells: 40 million Mesenchymal Stem Cells (MSCs) and 80 million Amniotic Fluid Stem Cells (hAFSCs) through one IV injection and two lumbar puncture injections, respectively. All three applications went well without adverse effects and no short-term or acute complications have been reported.
Device mapping and therapy were carried out after surgery for 35 days, then patient was discharged.
Results
- Motor Functions
- Sensory Functions
- Autonomic Functions
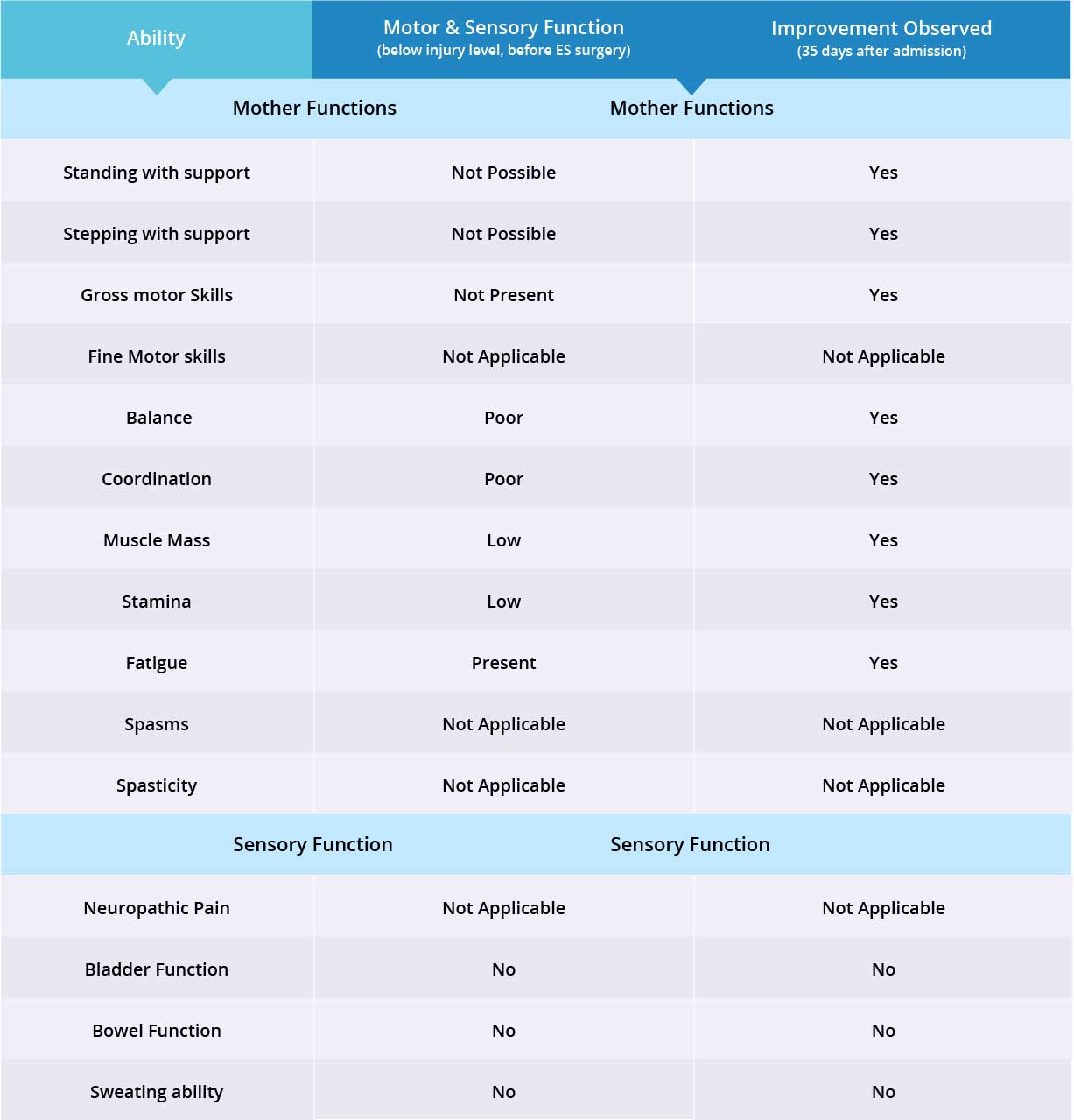
Patient’s gross motor skills have improved significantly, including ankle, hip and knee flexion, and knee extension (kicking out) when epidural stimulation device is switched on. Patient has good static and dynamic sitting balance and is able to touch his feet and put on his shoes.
Patient’s static standing balance is poor and dynamic standing balance non-existent. Muscle mass and endurance were improved upon discharge, and with physical therapy the patient was able to lose 20 kg.
Patient did not require a hoist while standing or during stepping exercises. Patient is able to stand at the parallel bar very well with no support. Patient is able to lock his knees with assistance, but is unable to keep them locked for long periods. Patient has good trunk control and bears weight equally on both legs while standing, but struggles to lock his hips.
During stepping exercises, patient was able to lift his feet very well. Patient is able to bear weight equally on both feet, but requires assistance with foot placement. Patient has good coordination in both legs when stepping.
Results Interpretation
Patient is paraplegic with normal upper limb function (fine motor skills), and does not experience spasms or spasticity, therefore 8 of 11 motor function areas were measured. Motor function improved in 8 out of 8 targeted areas when the epidural stimulation device was switched on.
Patient does not suffer from neuropathic pain, therefore 3 of 4 sensory function areas were measured. Patient has not experienced any changes in the three measured sensory function areas, but more feedback will be collected after 3 months to note any improvements made by stem cell treatment. Overall, improvements were observed in 8 out of 11 targeted motor and sensory function areas
