Case Study
Patient O,
Male, American
Table of Contents
Patient Overview
Age at time of treatment: 18 – 25
Injury Level: C6
Treatment Received: Stem Cells, Epidural Stimulation
Location of Treatment: Thailand
Time between injury and treatment: 2 – 5 years
Date of Surgery: 30/05/2017
Date of Discharge: 08/07/2017
Condition on Admission
Patient sustained a C6 burst fracture that was treated with emergent anterior corpectomy and C5-C7 spinal fusion in October, 2014. Patient noticed improvements from the procedures in core muscles, but is still very weak.
Patient is a C6 incomplete quadriplegic, therefore he has limited fine motor skills, but no motor function in lower limbs. He has minimal sensory functions and suffers from neurogenic bowel and bladder as well as spasms and spasticity. Patient takes Neurontin and Gabapentin for neuropathic pain, but does not feel any relief from the medication.
Previous Therapies & Treatments
Patient received 3 months of inpatient rehabilitation, followed by 18 months of outpatient aqua therapy and locomotor training.
Patient sustained a C6 burst fracture that was treated with emergent anterior corpectomy and C5-C7 spinal fusion in October, 2014. Patient noticed improvements from the procedures in core muscles, but is still very weak.
Patient is a C6 incomplete quadriplegic, therefore he has limited fine motor skills, but no motor function in lower limbs. He has minimal sensory functions and suffers from neurogenic bowel and bladder as well as spasms and spasticity. Patient takes Neurontin and Gabapentin for neuropathic pain, but does not feel any relief from the medication.
Verita Neuro Treatment Received
After a spinal MRI scan and comprehensive blood work, patient underwent laminectomy and implantation of the epidural stimulation device on June 2, 2017. The device is the ‘Medtronic Restore Advance 16-electrode MRI Compatible Device’. The surgery was completed without significant adverse effects and the surgical wound healed normally. No serious complications were reported during the postoperative hospital stay.
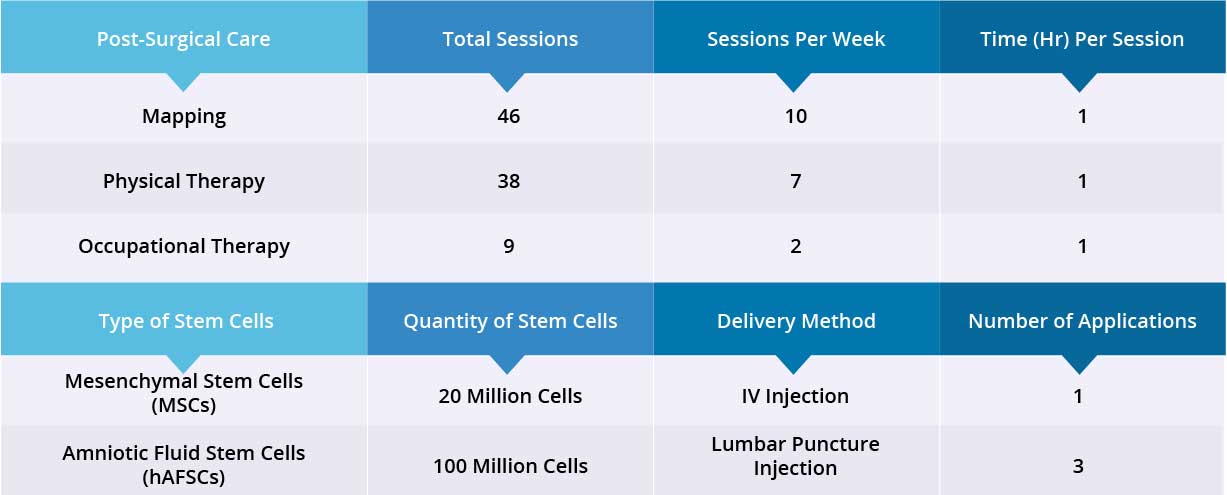
After the epidural stimulation surgery, patient received 46 mapping sessions, 38 physical therapy and 9 occupational therapy sessions. Patient also received 120 million Mesenchymal Stem Cells (MSCs) through three lumbar puncture injections and one IV injection. All four stem-cell treatments went well without adverse effects and no short-term or acute complications have been reported.
Device Mapping and therapy were carried out after surgery for 35 days, then patient was discharged.
After a one year follow up, the patient is very satisfied with the epidural stimulation device and the stem cell treatment he received at Verita Neuro. Patient states: “The epidural stimulation has improved my quality of life. As a result, I feel and look happier and have more function. I am glad I went.”
Results
- Motor Functions
- Sensory Functions
- Autonomic Functions
Patient is able to take assisted steps with the use of a hoist and walking frame. Patient is able to lift his feet by himself and his coordination has improved in both legs, but he requires assistance in foot placement and knee locking.
1 Year Post-Treatment
Patient’s gross motor functions such as flexing and extending his hips and knees have improved moderately. The programs provided by Verita Neuro for gross motor functions are still working very well. On a scale of 1-5, 1 being worse than before surgery and 5 being significantly better, patient rates his gross motor functions as a 3 — Moderate improvement.
Patient has noticed moderate improvement when standing with support and he is able to stand for longer periods of time. Patient is able to lock his knees by himself on certain occasions and his standing balance is good when he receives arm support. Patient has moderate trunk control while standing. On a scale of 1-5, 1 being worse than before surgery and 5 being significantly better, patient rates his standing with support as a 3 — Moderate improvement.
Patient has noticed significant improvement during stepping exercises when using a walking frame. He sometimes requires assistance in foot placement but is able to lift his legs by himself while taking steps. He is also able to lock his knees on his own when taking steps despite sometimes requiring assistance. Patient has moderate trunk control. The coordination of his legs when taking steps is the same since he left Verita Neuro after his 35-day post-operative rehabilitation.
Patient states that due to being away for his studies he is unable to do as many standing and stepping exercises as his therapists would like, but this will resume once he is back home. Patient has noticed slight improvement in muscle mass, with the biggest gains in his quadriceps and calves. Patient has also noticed moderate improvement in his stamina and the reduction of fatigue.
1 Year Post-Treatment
Patient states that spasms and spasticity have increased slightly since he was discharged from Verita Neuro. However, patient is not taking any medication for spasms or spasticity. Patient was provided with an overnight program on the epidural stimulation device to help ease spasms and spasticity, but does not use it due to the sensations it creates when he is trying to fall asleep. He describes this as not painful but distracting.
Patient has not noticed any improvement in bladder function, but has noticed slight improvement in bowel function. Patient has not regained sensation in bowel function, but has noticed improvement in bowel control and is able to empty his bowel faster.
Patient has noticed mild improvement in sweating ability. Previously, patient was not able to sweat below his injury level, but now he is able to sweat slightly in his arms and elbows.
Patient also reported that his blood pressure has improved with the epidural stimulation device switched on. Patient has orthostatic hypotension and states that since his surgery, his blood pressure has increased to normal levels, allowing him to carry out his daily activities without feeling light headed.
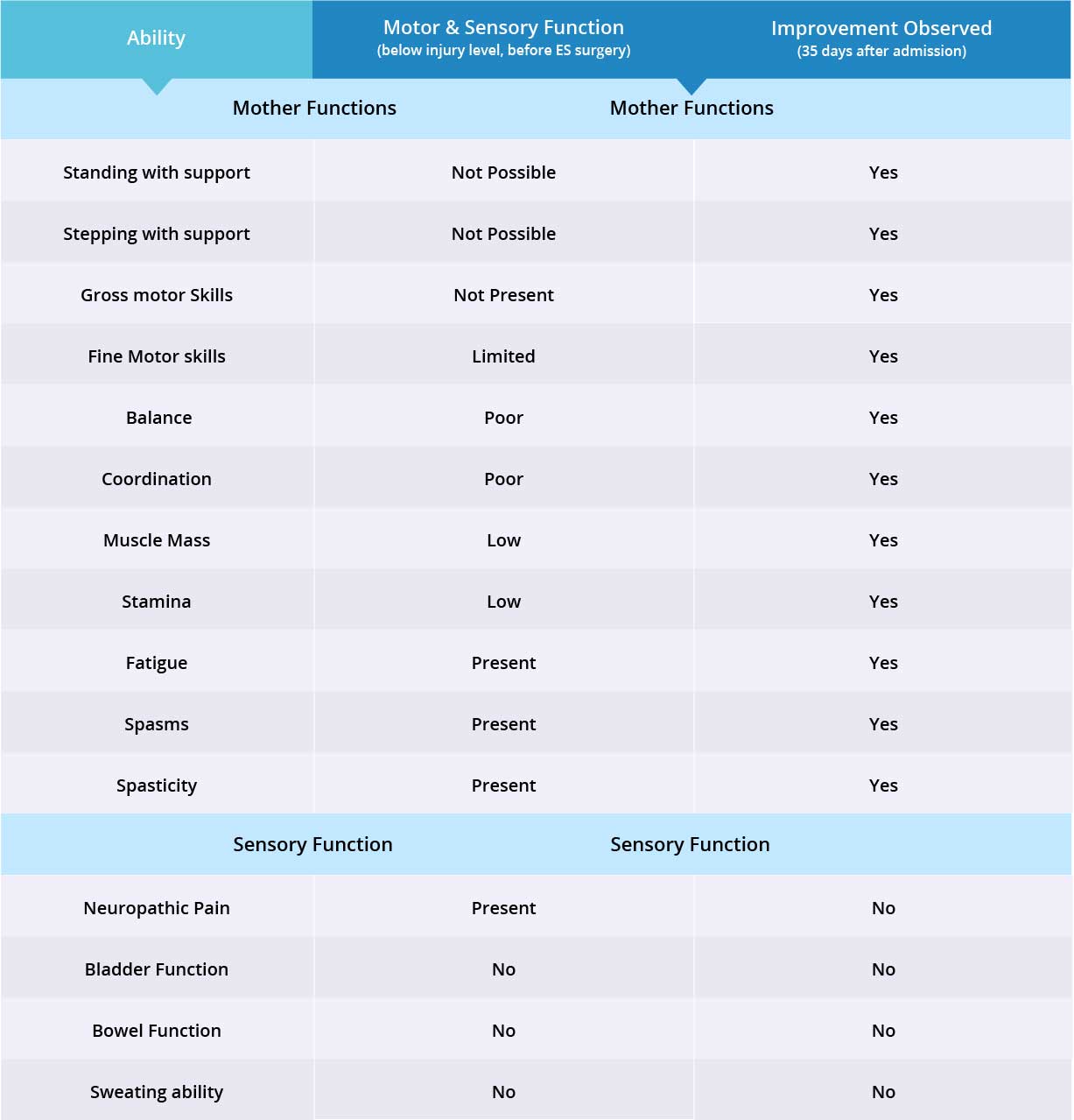
Improvements are monitored in 15 targeted areas: 11 Motor areas and 4 Sensory areas. However, the number of targeted areas may vary depending on patient’s condition prior to admission. If patient does not experience symptoms in certain Motor/Sensory functions, or is not impaired in a specific targeted area prior to surgery, it is excluded from the report (Not Applicable). If there is progress in any given area — either mild, moderate, or significant — it is measured and reported as positive (“Yes”). No improvement, the existence of pain or spasms, or an inability to perform a measured function is reported as “No”.
Results Interpretation
In this patient, 15 of 15 motor and sensory functions were targeted. Overall, improvements were recorded in 11 out of 15 targeted motor and sensory function areas. Motor function improved in 11 out of 11 targeted areas when the epidural stimulation device was switched on, but there were no improvements in sensory function. More feedback will be collected to note any improvements made by the stem cell treatments.

