Case Study
Patient P,
Male, American
Table of Contents
Patient Overview
Age at time of treatment: 36 – 45
Injury Level: C5, C6
Treatment Received: Epidural Stimulation
Location of Treatment: Thailand
Time between injury and treatment: 5 – 15 years
Date of Surgery: 12/08/2017
Date of Discharge: 18/09/2018
Condition on Admission
Patient suffered an incomplete C5-C6 spinal cord injury on June 22, 1995. Patient is quadriplegic, but is able to move his arms without any movement of his fingers. He is able to sit upright, but can keep his balance for only a few minutes without support. Patient does have bladder and bowel sensation, but with very little control. Patient does not have neuropathic pain.
Previous Therapies & Treatments
Patient received stem cells in 2006 (twice), 2008, and 2009 in a facility in China. Past surgeries include spinal decompression in 2008 and surgery for tendons and ligaments in both hands in 2014. He performs physical and occupational therapy 5 times per week, and in 2016 received several sessions of physical therapy and acupuncture with Verita Neuro.
Patient suffered an incomplete C5-C6 spinal cord injury on June 22, 1995. Patient is quadriplegic, but is able to move his arms without any movement of his fingers. He is able to sit upright, but can keep his balance for only a few minutes without support. Patient does have bladder and bowel sensation, but with very little control. Patient does not have neuropathic pain.
Verita Neuro Treatment Received
After a spinal MRI scan, EMG, and comprehensive blood work, patient underwent laminectomy and implantation of the epidural stimulation device on August 12, 2017. The device is the ‘Medtronic Restore Advance 16-electrode MRI Compatible Device’. The surgery was completed without issue and no serious complications were reported during the postoperative hospital stay. Surgical wounds healed normally and no spinal cord or superficial wound infection was reported.
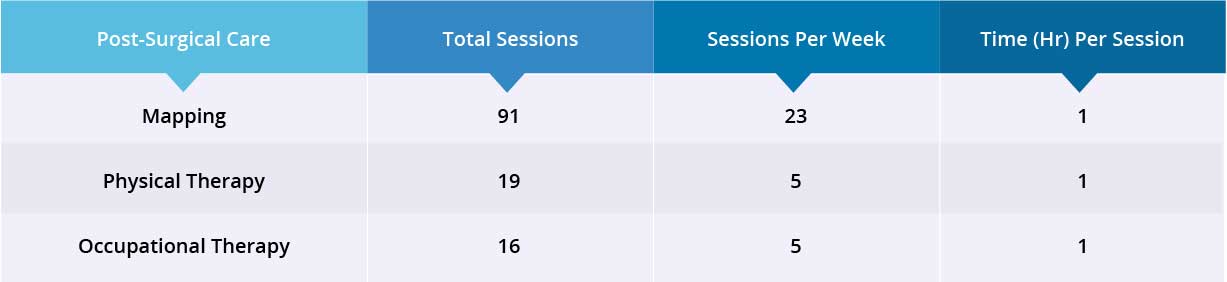
After epidural stimulation surgery, patient received 91 mapping sessions, 19 physical therapy sessions, and 16 occupational therapy sessions.
Device mapping and rehabilitation was carried out after surgery for 35 days, then patient was discharged.
Results
- Motor Functions
- Sensory Functions
- Autonomic Functions
Patient’s stamina and muscle mass increased noticeably. Muscle mass increased up to 2.5 cm in limbs and an increase in abdominal strength was also reported. Reduction in fatigue was noticeable.
Spasms and spasticity were also reduced.
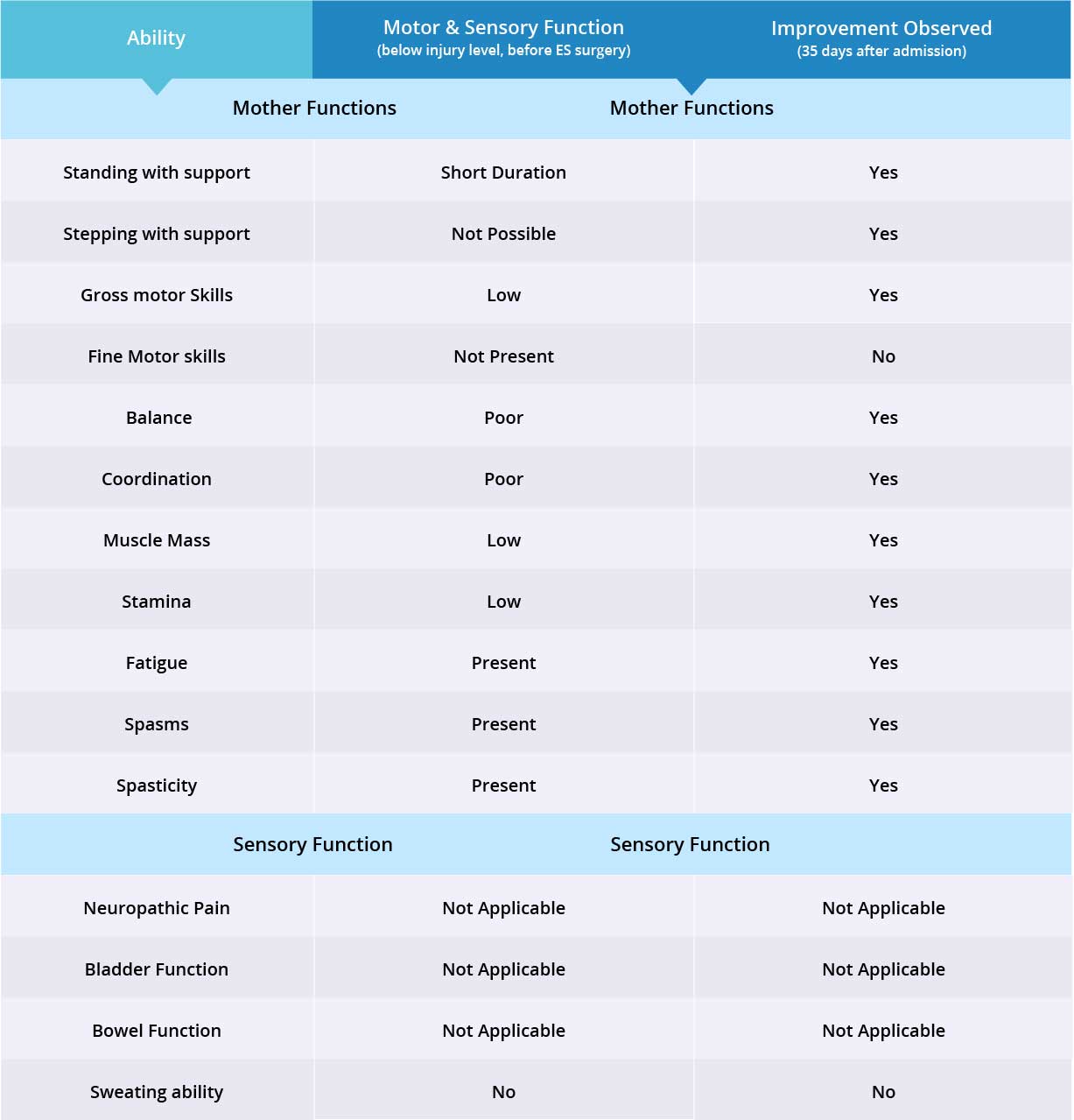
Improvements are monitored in 15 targeted areas: 11 Motor areas and 4 Sensory areas. However, the number of targeted areas may vary depending on patient’s condition prior to admission. If patient does not experience symptoms in certain Motor/Sensory functions, or is not impaired in a specific targeted area prior to surgery, it is excluded from the report (Not Applicable). If there is progress in any given area — either mild, moderate, or significant — it is measured and reported as positive (“Yes”). No improvement, the existence of pain or spasms, or an inability to perform a measured function is reported as “No”.
Results Interpretation
For this patient, 12 areas instead of 15 were reviewed. The patient’s prior stem cell injections resulted in improving bowel and bladder sensation, which was not measured by Verita Neuro. The patient also did not experience neuropathic pain so improvements there were also not measured.
The primary treatment was focused on regaining motor function and improved in 10 out of 11 targeted areas with the Epidural Stimulation device switched on. Patient improved in 11/12 targeted motor and sensory functions. Sensory Function improved in the only targeted area. Overall improvement in both motor and sensory functions was 11/12.
